Pharmaceutical Open Innovation Test Bed for Enabling Nano-pharmaceutical Innovative Products
Official website: https://www.phoenix-oitb.eu/
Project acronym: PHOENIX
Project duration: 1st March 2021 – 28th February 2025
Project coordinator: MyBiotech, Germany
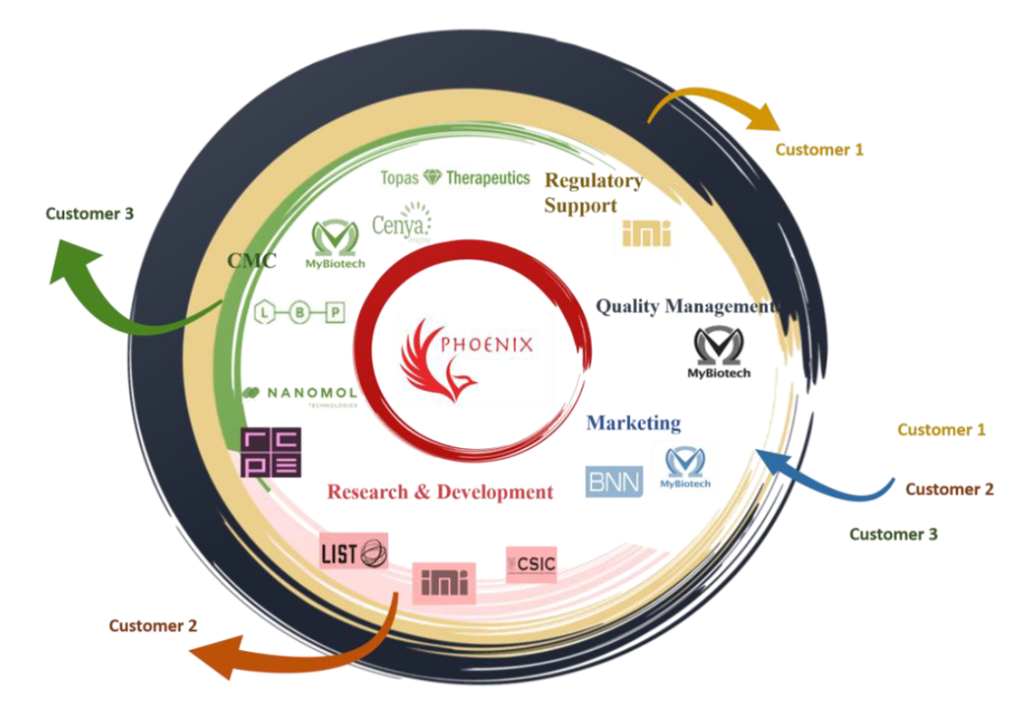
Our role: Leader for Work Package 5 – Regulatory support
Funded by: European Union Horizon 2020 Programme (H2020) under grant agreement nº 953110
Total budget: 11.118.925,26 EUR
Abstract: Nano-pharmaceuticals have the potential to drive the scientific and technological uplift, offering great clinical and socioeconomic benefits to the society in general, industry and key stakeholders and patients. Nevertheless, affordable and advanced testing, manufacturing facilities and services for novel nano-pharmaceuticals are main prerequisites for successful implementation of these advances to further enhance the growth and innovation capacity. The establishment of current good manufacturing practice (cGMP) in nano-pharmaceutical production at large scale is the key step to transfer successfully the nano-pharmaceuticals from bench to bedside (from lab to industrial scale). Due to the lack of resources to implement GMP manufacturing at-site, the upscaling and production of innovative nano-pharmaceuticals is still challenging to main players of EU nanomedicine market, start-ups and SMEs. To allow successful implementation of the nanopharmaceuticals in the nanomedicine field, there is an urgent need to establish science- and regulatory-based Open Innovation Test Bed (OITB). Phoenix aims to enable the seamless, timely and cost-friendly transfer of nano-pharmaceuticals from lab bench to clinical trials by providing the necessary advanced, affordable and easily accessible Phoenix-OITB. Phoenix-OITB will offer a consolidated network of facilities, technologies, services and expertise for all the technology transfer aspects from characterization, testing, verification up to scale up, GMP compliant manufacturing and regulatory guidance. Phoenix-OITB will develop and establish new facilities and upgrade existing ones to make them available to SMEs, starts-up and research laboratories for scale-up, GMP production and testing of nano-pharmaceuticals. The services and expertise provided by the OITB will include production and characterization under GMP conditions, safety evaluation, regulatory compliance and commercialization boost.
Project partners:
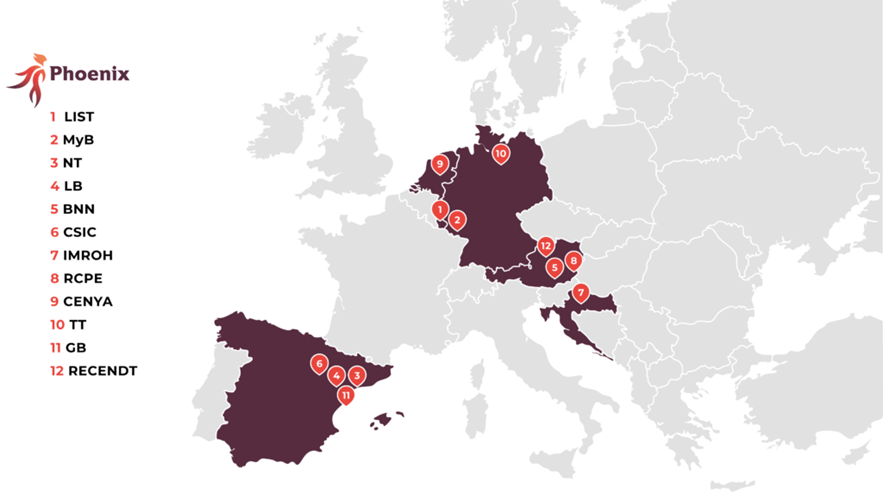
MyBiotech GmbH (Germany) – Coordinator
Nanomol Technologies SL (Spain)
LeanBio SL (Spain)
BioNanoNet Forschungsgesellscchaft mbH (Austria)
Luxembourg Institute of Science and Technology (Luxembourg)
Agencia Estatal Consejo Superior Deinvestigaciones Científicas (Spain)
Institute for Medical Research and Occupational Health (Croatia)
Research Center Pharmaceutical Engineering GmbH (Austria)
Cenya Imaging B.V. (Netherlands)
Topas Therapeutics GmbH (Germany)
Grace Bio S.L. (Spain)
RECENDT (Austria)